12. Head and neck cancer
12.1 Summary
The category of head and neck cancer incorporates cancers at 17 separate sites in the mouth, pharynx, larynx, middle ear and nasal sinuses (see Table 2.1). Head and neck cancer was the ninth most common cancer in Ireland, accounting for 1.6% of all malignant neoplasms, excluding non-melanoma skin cancer, in women and 4.0% in men (Table 12.1). The average number of new cases diagnosed each year was 170 in women and 438 in men. During 1995-2007, the number of new cases diagnosed increased by approximately 1% per annum.
The risk of developing head and neck cancer up to the age of 74 was 1 in 209 for women and 1 in 67 for men and was slightly higher in NI than in RoI for women. At the end of 2008, 568 women and 1,293 men aged under 65, and 600 women and 1,462 men aged 65 and over, were alive up to 15 years after their head and neck cancer diagnosis.
Table 12.1 Summary information for head and neck cancer in Ireland, 1995-2007
Ireland | RoI | NI | ||||
females | males | females | males | females | males | |
% of all new cancer cases | 1.2% | 2.9% | 1.1% | 2.7% | 1.4% | 3.2% |
% of all new cancer cases excluding non-melanoma skin cancer | 1.6% | 4.0% | 1.5% | 3.9% | 1.9% | 4.3% |
average number of new cases per year | 170 | 438 | 105 | 294 | 65 | 144 |
cumulative risk to age 74 | 0.5% | 1.5% | 0.4% | 1.5% | 0.6% | 1.5% |
15-year prevalence (1994-2008) | 1168 | 2755 | 724 | 1765 | 444 | 990 |
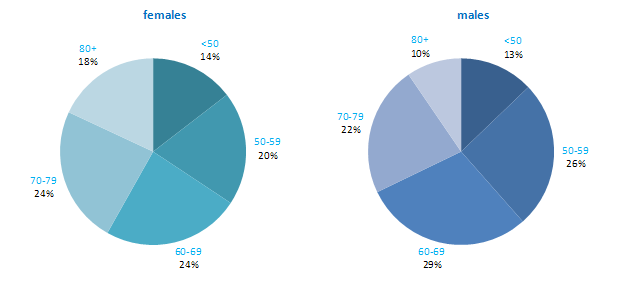
The age distribution for head and neck cancer showed that men tended to be diagnosed at an earlier age than women—68% of men were aged under 70 years, compared to 58% of women (Figure 12.1). Almost twice the percentage of women presented at 80 years and over compared to men (18% .v. 10%). The pattern of age at diagnosis was similar in RoI and in NI.
Figure 12.1 Age distribution of head and neck cancer cases in Ireland, 1995-2007, by sex
12.2 International variations in incidence
The incidence of head and neck cancer in women was low, with age-standardised rates ranging from 2.1 per 100,000 (in Japan) to 6.3 per 100,000 (in Demark) (Figure 12.2). Variation in rates was greater for men, with rates highest in Spain and Portugal, and lowest in Japan and New Zealand. Rates in RoI and NI were close to the median of the countries examined for men; NI ranked fifth highest of the 21 countries for women.
Figure 12.2 Estimated incidence rate per 100,000 in 2008 for selected developed countries compared to 2005-2007 incidence rate for RoI and NI: head and neck cancer | |
| females | males |
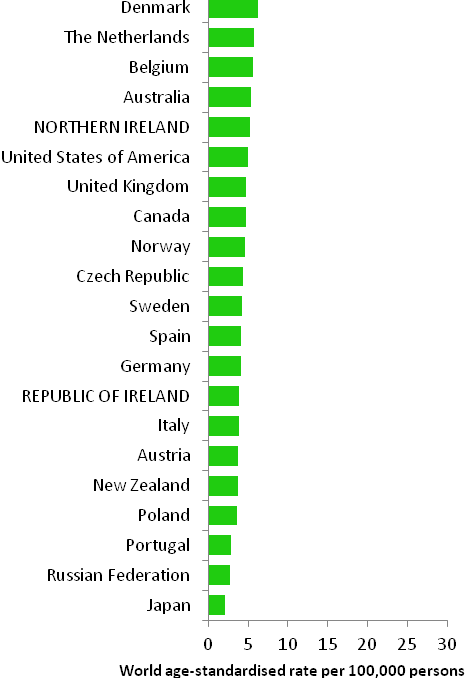 | 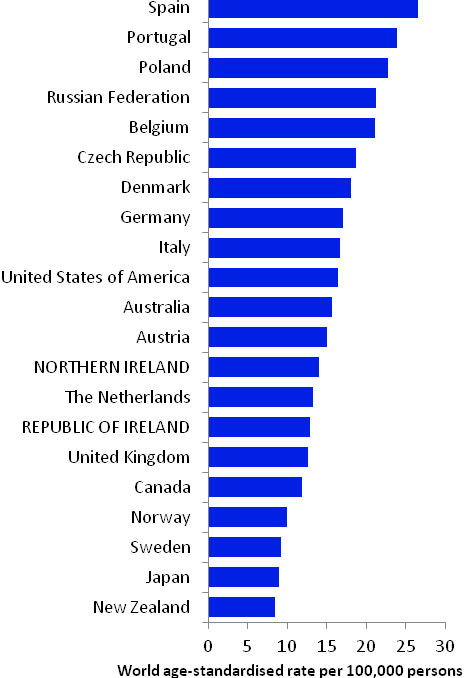 |
Source: GLOBOCAN 2008 (Ferlay et al., 2008) (excluding RoI and NI data, which is derived from Cancer Registry data for 2005-2007) NOTE: Head and neck cancer was defined in GLOBOCAN 2008 by ICD10 codes C00-C14 and C32 but in this atlas we have used codes C01-C14 and C30-C32 (see Table 2.1). The incidence rates shown for NI and RoI in Figure 12.2 use the GLOBOCAN definition, and are consequently slightly inconsistent with data in the rest of this chapter. | |
12.3 Risk factors
Table 12.2 Risk factors for head and neck cancer, by direction of association and strength of evidence
Increases risk | Decreases risk | |
Convincing or probable | Tobacco smoking1 | |
| Smokeless tobacco1 | |
| Involuntary (passive) smoking1,2 | |
| Alcohol1 | |
| Infection with human papilloma viruses (HPV)3 | |
| Low socio-economic status4 | |
| Family history5 | |
Possible | Body leanness/being underweight6,7 | Fruit8 |
Non-starchy vegetables8 | ||
Foods containing carotenoids8,9 | ||
Coffee10 | ||
1 Secretan et al., 2009; 2 Lee et al., 2008; 3 International Agency for Research on Cancer, 2011b; 4 Faggiano et al., 1997; 5 Negri et al., 2009; 6 Gaudet et al., 2010; 7 Lubin et al., 2011; 8 World Cancer Research Fund / American Institute for Cancer Research, 2007; 9 carotenoids are found in vegetables, particularly those which are red or orange; 10 Turati et al., 2011 | ||
More than 70% of head and neck cancers are considered to be due to tobacco and alcohol (Table 12.2; Hashibe et al., 2009). Tobacco smoking, and use of smokeless tobacco products, such as chewing tobacco or snuff, are causally related to cancer at many of the specific sites within this group. Risk increases with duration of smoking and number of cigarettes smoked, and falls with increasing time since quitting. Risk, particularly of laryngeal and pharyngeal cancers, is probably also increased in those who have never smoked themselves, but have a long duration of involuntary smoking (passive smoking) exposure at home or work. A causal relationship with alcohol intake is also clearly established. Compared to non- or occasional drinkers, light drinkers have a modest increased risk of oral and pharyngeal cancers, while the risk in heavy drinkers is increased by 5-fold for oral cancers and 7-fold for pharyngeal cancers (Turati et al., 2010).
Overall, individuals with one or more first-degree relatives affected with head and neck cancer have a modest raised risk of developing the disease themselves, although the combination of a positive family history and use of alcohol and tobacco confers a much higher risk. Risk of most head and neck cancers is higher in those of lower socio-economic status, probably reflecting social class variations in exposure to tobacco and, perhaps also, alcohol.
Evidence of infection with human papilloma viruses (HPV) has been found in the oral cavity and larynx. Moreover, sexual behaviours that have previously been associated with HPV infection, such as earlier age at sexual debut and more sexual partners, have also been associated with increased risk of head and neck cancer (Heck et al., 2010). Consequently, the International Agency for Research on Cancer has concluded that various strains of HPV are causally implicated in some head and neck cancers. Notably, HPV16 is considered a causal agent for cancers of the oral cavity, oropharynx and tonsil, and is likely to also be involved in the aetiology of laryngeal cancer. However, the natural history of oral HPV infection remains unclear.
Systematic reviews suggest that higher intake of fruit and vegetables (non-starchy or carotenoid-rich) may be associated with decreased risk of head and neck cancer. Similarly, risk of cancers of the oral cavity and pharynx, but not the larynx, may be lower in those who consume more coffee. Body leanness and being underweight have been associated with increased risk of head and neck cancer, and overweight and obesity with reduced risk, but the possibility of reverse causality cannot be excluded.
12.4 Small geographic area characteristics and cancer risk
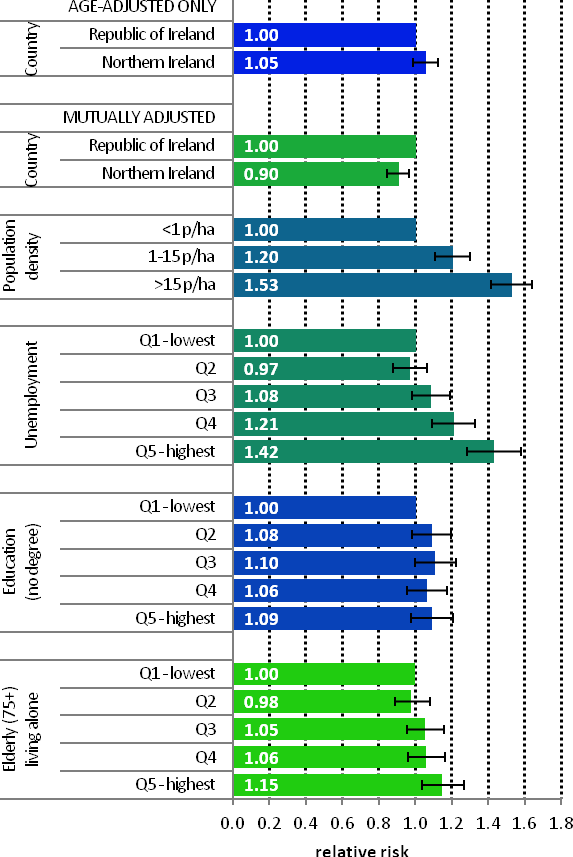
Figure 12.3 Adjusted relative risks (with 95% confidence intervals) of head and neck cancer by socio-economic characteristics of geographic area of residence: males | MalesThe risk of head and neck cancer among men was slightly higher in NI (Figure 12.3). However, after adjustments for population density and socio-economic factors, the risk was lower in NI than RoI (RR=0.90, 95%CI=0.84-0.97). The risk of male head and neck cancer increased considerably with increasing population density. Men resident in areas with 1-15 p/ha had a 20% greater risk than men resident in the least densely populated areas, while men resident in the most densely populated areas had a 53% greater risk. Similarly, the risk of head and neck cancer increased with increasing unemployment in the area of residence. In particular, men resident in the areas of highest unemployment had a 42% greater risk of head and neck cancer than those in the areas of lowest unemployment. However, lower educational attainment was not associated with head and neck cancer among men. Areas with the highest proportion of elderly living alone had a 15% elevated risk of male head and neck cancer compared to areas with the lowest proportion. |
Figure 12.4 Adjusted relative risks (with 95% confidence intervals) of head and neck cancer by socio-economic characteristics of geographic area of residence: females
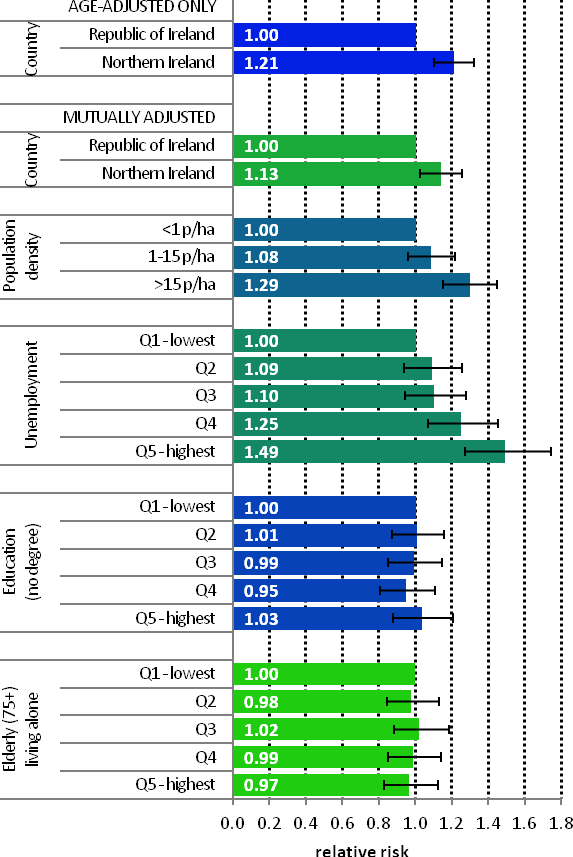
| FemalesThe age-adjusted risk of head and neck cancer for women in NI was 21% higher than in RoI (Figure 12.4). After adjustments for population density and socio-economic factors the risk in NI was still greater than in RoI but to a slightly lesser degree (RR=1.13, 95%CI=1.03-1.26). The association between female head and neck cancer and population density was weaker than that for men. Compared to the least densely populated areas head and neck cancer risk was 29% greater in the most densely populated areas. As with men, there was a positive association between head and neck cancer risk among women and unemployment. Women resident in the areas of highest unemployment had a 49% greater risk of head and neck cancer than those in the areas of least unemployment. There was no association between female head and neck cancer and education or the proportion of elderly living alone in an area. |
12.5 Mapping and geographical variation
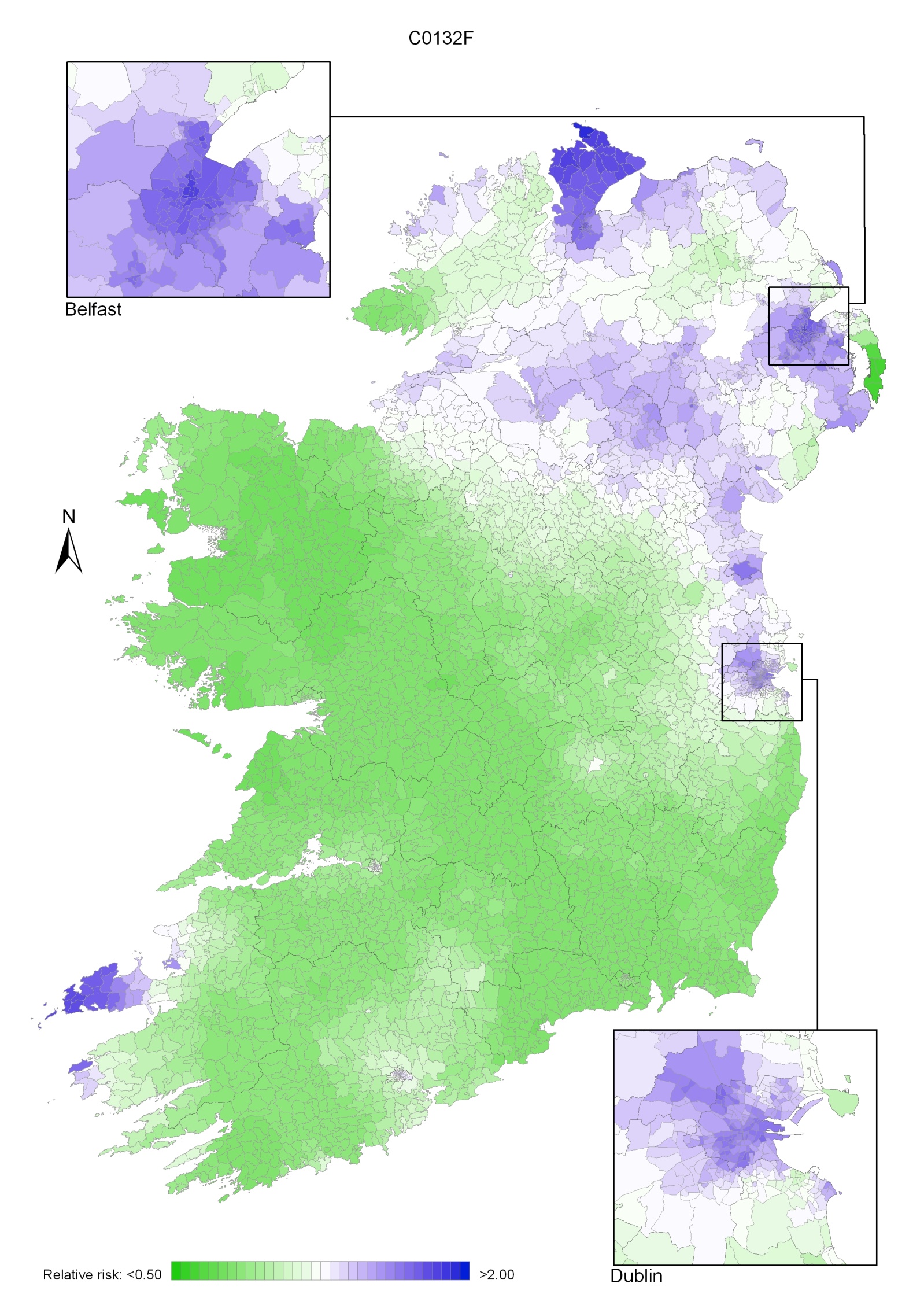
Due to the much higher incidence of head and neck cancer in men, the geographical pattern for both sexes (Map 12.1) was similar to that for men only.
Areas of high relative risk for men were scattered throughout the country—along the western seaboard, around Cork, Kerry, Tipperary South, Clare, Limerick, Dublin city and north Dublin, Belfast, Moyle, Larne, Cookstown, Derry, Newry and Mourne, Longford, Galway and Mayo (Map 12.2).
There was a quite different pattern of geographical variation for women with one large area of higher relative risk from north Leitrim/south Donegal to the east coast of NI, and covering most of the southern half of NI and the border counties in RoI. Other areas of higher risk were in Dublin city, north Dublin, the Inishowen peninsula in Donegal, the Dingle peninsula in Kerry and along the northern coast of NI from Derry to Moyle (Map 12.3).
Map 12.1 Head and neck cancer, smoothed relative risks: both sexes
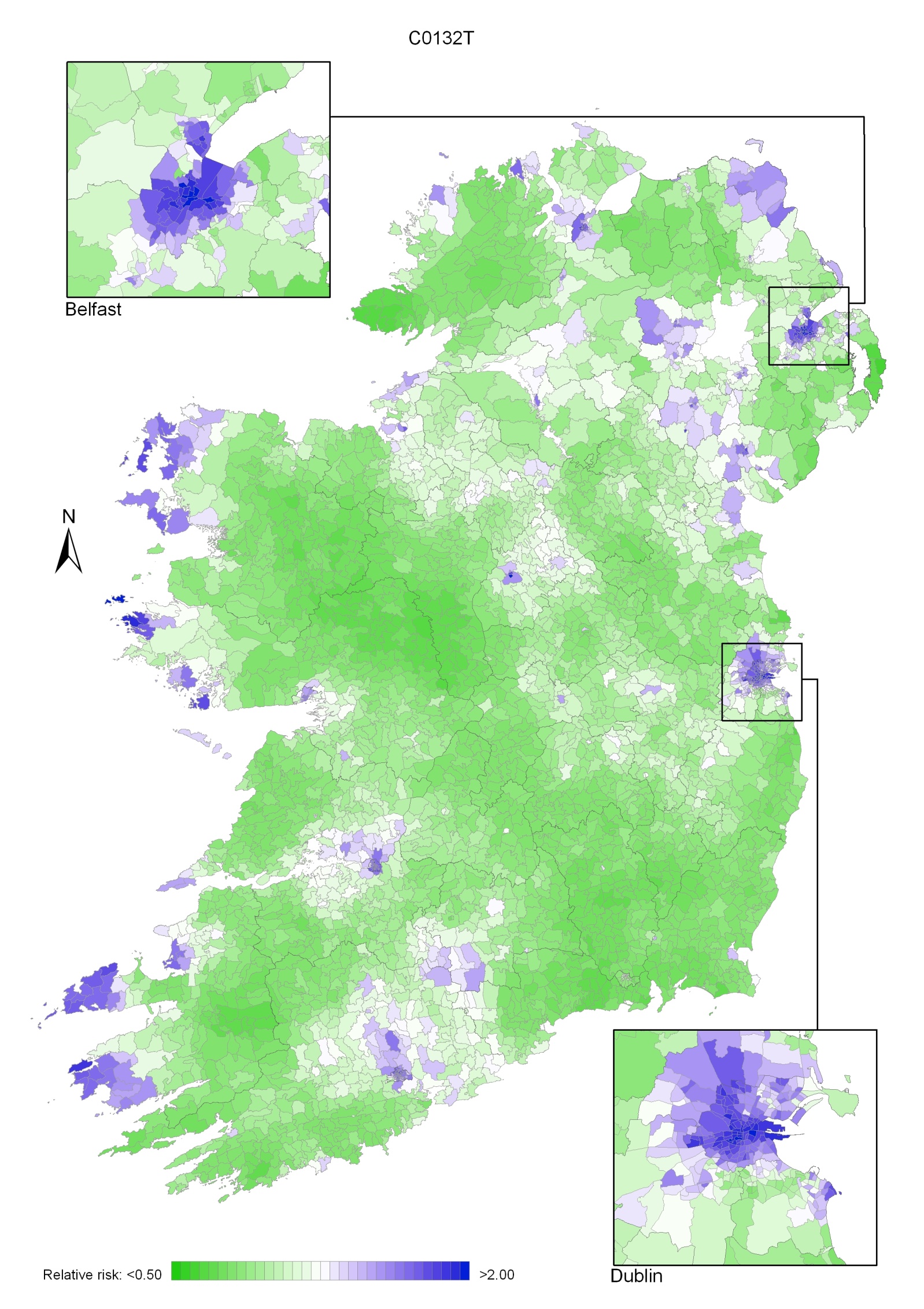
Map 12.2 Head and neck cancer, smoothed relative risks: males
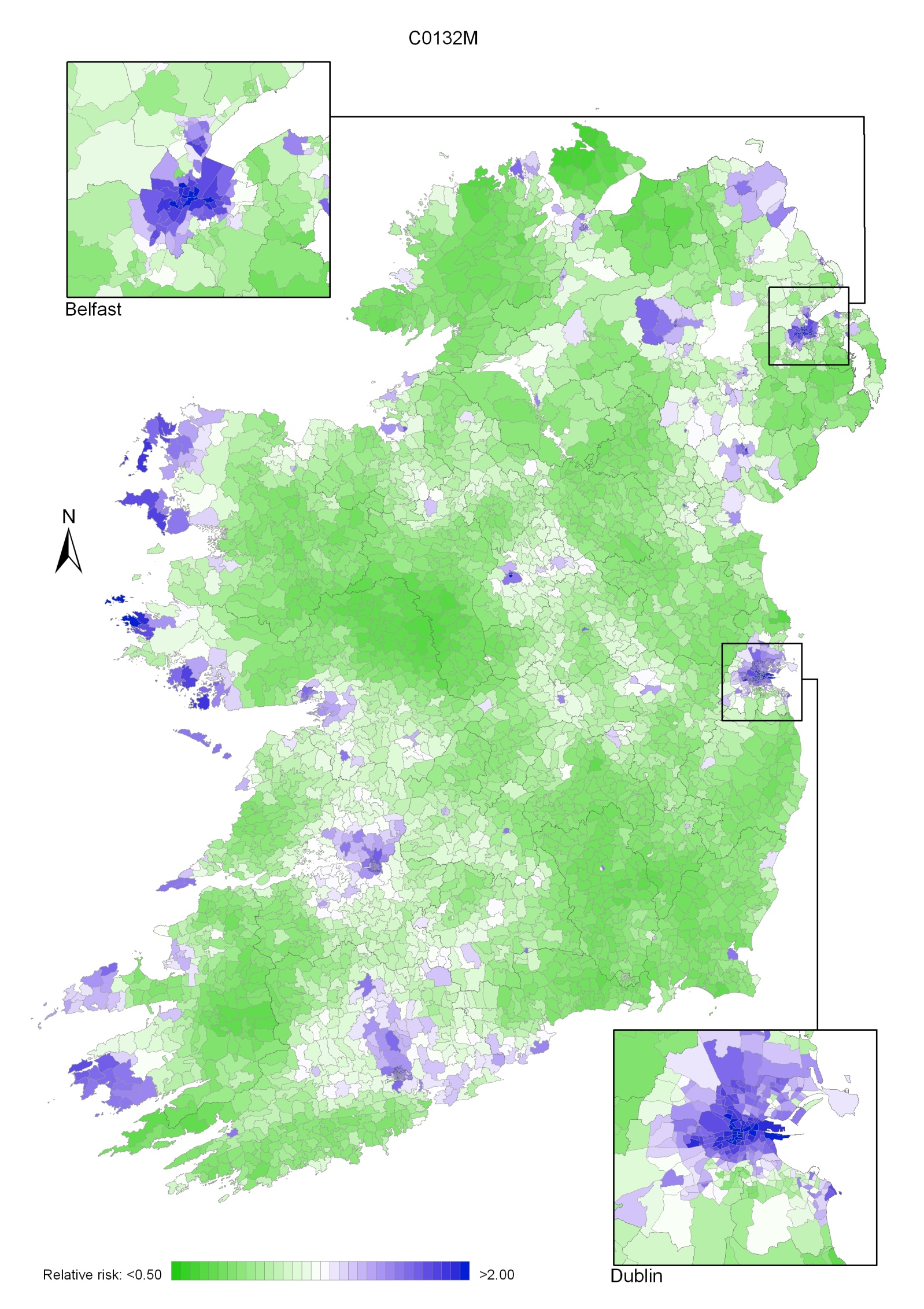
Map 12.3 Head and neck cancer, smoothed relative risks: females