Cancer of the uterus is classified by ICD-10 into three sites—cancer of the cervix uteri (cervical cancer; see Chapter 20), cancer of the corpus uteri (uterine cancer; discussed in this chapter), and cancer of the uterus, part unspecified. “Part unspecified” cases make up less than 5% of all cancers of the uterus and are not considered here.
Cancer of the corpus uteri (uterine cancer) was the sixth most common cancer in women in Ireland, accounting for 3.9% of all malignant neoplasms, excluding non-melanoma skin cancer, in women (Table 19.1). The average number of new cases diagnosed each year was 403. During 1995-2007, the number of new cases diagnosed per year increased by 7% in NI and 3% in RoI.
The risk of developing uterine cancer up to the age of 74 was 1 in 77 and was slightly higher in NI than in RoI. At the end of 2008, 1,756 women aged under 65, and 2,419 aged 65 and over, were alive up to 15 years after diagnosis.
Table 19.1 Summary information for uterine cancer in Ireland, 1995-2007
Ireland | RoI | NI | |
% of all new cancer cases | 2.9% | 2.7% | 3.2% |
% of all new cancer cases excluding non-melanoma skin cancer | 3.9% | 3.7% | 4.2% |
average number of new cases per year | 403 | 258 | 145 |
cumulative risk to age 74 | 1.3% | 1.2% | 1.4% |
15-year prevalence (1994-2008) | 4175 | 2623 | 1552 |
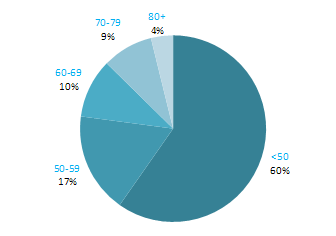
Almost 80% of cases of uterine cancer were diagnosed between 50 and 79 years of age, with 27% presenting between 50 and 59 years, 30% between 60 and 69 years and 22% between 70 and 79 years (Figure 19.1). Only 11% of cases were diagnosed in women aged less than 50, with a further 10% diagnosed in those aged 80 and older.
Figure 19.1 Age distribution of cases of uterine cancer in Ireland, 1995-2007
Rates of uterine cancer varied considerably among developed countries, with rates highest in the Czech Republic and Norway and lowest in Japan and Portugal (Figure 19.2). In RoI the incidence rate of the disease was low compared to other countries, while in NI the rate was slightly higher than the median. Variation between countries in the percentage of cases assigned to “uterus, part unspecified” (which are not included in the data below) may account for some of the international variation.
Figure 19.2 Estimated incidence rate per 100,000 in 2008 for selected developed countries compared to 2005-2007 incidence rate for RoI and NI: uterine cancer |
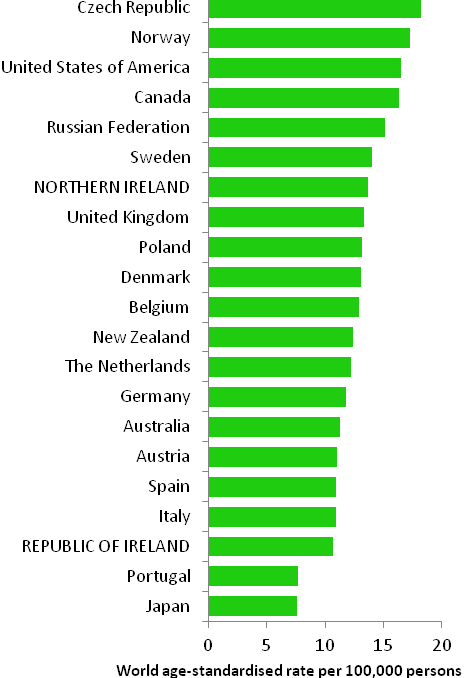 |
Source: GLOBOCAN 2008 (Ferlay et al., 2008) (excluding RoI and NI data, which is derived from Cancer Registry data for 2005-2007) |
Table 19.2 Risk factors for uterine cancer, by direction of association and strength of evidence
Increases risk | Decreases risk | |
Convincing or probable | Hormone replacement therapy1,2 | Oral contraceptives2,18 |
| Tamoxifen2 | Physical activity4 |
| Nulliparity3 | |
| Greater body fatness/abdominal fatness4,5 | |
| Diabetes6,7 | |
| Lynch syndrome (hereditary nonpolyposis colorectal cancer (HNPCC))8,9 | |
| Family history of cancer of the corpus uteri10,11,12 | |
Possible | Early menarche13 | Breast feeding19,20 |
Late natural menopause13 | Non-starchy vegetables4,21 | |
Polycystic ovary syndrome14 | Soya or soya food products22,23 | |
Red meat4,15 | Foods containing fibre24 | |
Diet with a high glycaemia load16,17 | ||
| ||
1 oestrogen-only formulations, and oestrogen-progestogen formulations where the progestogen is taken less than 15 days in the month; 2 International Agency for Research on Cancer, 2011a; 3 Dossus et al., 2010; 4 World Cancer Research Fund / American Institute of Cancer Research, 2007; 5 Crosbie et al., 2010 ; 6 Friberg et al., 2007; 7 Noto et al., 2010; 8 Bonadona et al., 2011; 9 Baglietto et al., 2010; 10 one or more first degree relative(s) with cancer of the corpus uteri; 11 Hemminki et al., 2005; 12 Lucenteforte et al., 2009; 13 Purdie and Green, 2001; 14 Jakimiuk and Issat, 2009; 15 includes beef, pork and lamb ; 16 glycaemic load is a ranking system for carbohydrate quantity and quality of food portions; 17 Mulholland et al., 2008; 18 combined oestrogen-progestogen pills; 19 Salazar-Martinez et al., 1999; 20 Newcomb & Trentham-Dietz, 2000; 21 includes broccoli, cabbage, carrots, cauliflower, celery, leeks, lettuce, onions, peas, peppers and spinach; 22 includes soya beans, edamame, tofu, soya milk; 23 Myung et al., 2009; 24 Bandera et al., 2007 | ||
Hormones play a major role in the aetiology of uterine cancer. A surge in incidence of these cancers in the USA in the late-1960s and mid-1970s led to the discovery of the role of hormone replacement therapy (Jick et al., 1980). Oestrogen-only formulations of hormone replacement therapy are now considered to be a causal agent and risk is also increased in women who use regimes which contain both oestrogen and progestogen, but in which the progestogen is taken for less than 15 days per month. Tamoxifen, a selective oestrogen receptor modulator used in the prevention and treatment of breast cancer, also causes uterine cancer. In contrast, use of combined oestrogen-progestogen oral contraceptives is protective. Risk is reduced by about half in women who have taken oral contraceptives, and the protective effect lasts for around two decades after cessation of use. However, it should be noted that these findings have generally been based on older high-dose oral contraceptives. In addition, reproductive factors associated with increased exposure to endogenous oestrogens may also influence risk. Women who have not had children (nulliparous) are at increased risk, while risk reduces with increasing number of full-term pregnancies. Risk may be raised in women with early menarche and late menopause, while breastfeeding may be protective. The strong and consistent association between body fatness and uterine cancer (60% increase in risk per 5 kg/m2 increase in body mass index) may also operate through effects on oestrogens and other hormones, such as insulin (World Cancer Research Fund / American Institute of Cancer Research, 2007). These effects are also a possible explanation for the observed relationship between higher levels of physical activity and reduced disease risk. As regards insulin levels more directly, there is a two to three-fold increased risk in diabetic individuals (both type 1 and type 2 diabetes).
Women who have one or more first-degree relatives affected by uterine cancer have a two-fold increased risk of developing the disease. Part of this is due to women in families affected by hereditary nonpolyposis colorectal cancer (HNPCC) syndrome, also known as Lynch syndrome. Depending on which genetic mutations they carry, these women have a 20-55% chance of developing uterine cancer in their lifetime.
Figure 19.3 Adjusted relative risks (with 95% confidence intervals) of uterine cancer by socio-economic characteristics of geographic area of residence | |
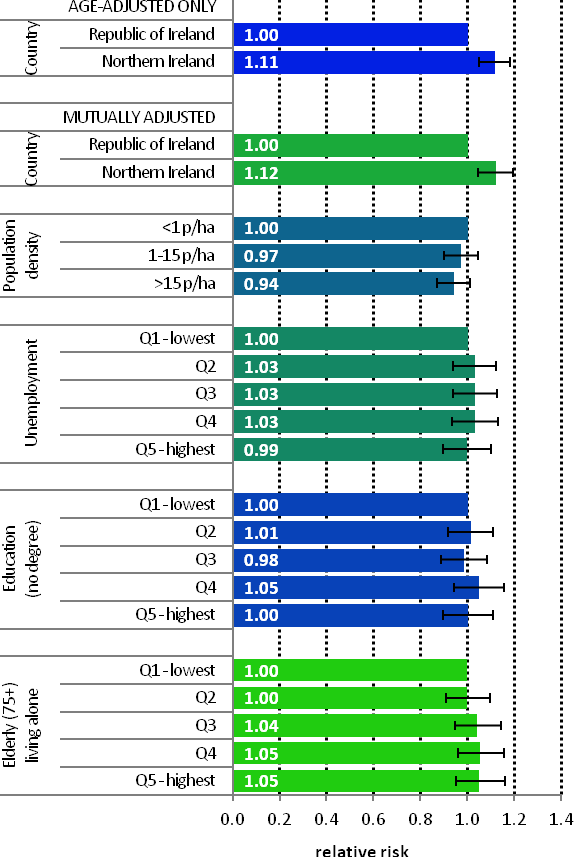 | Uterine cancer was more common in NI than RoI in 1995-2007 (RR=1.11, 95%CI=1.05-1.18), a difference not explained by variations in population density or small-area socio-economic characteristics (Figure 19.3) Other than this difference, there was minimal variation in uterine cancer risk across Ireland, with no significant relationship to population density or small-area socio-economic characteristics. |
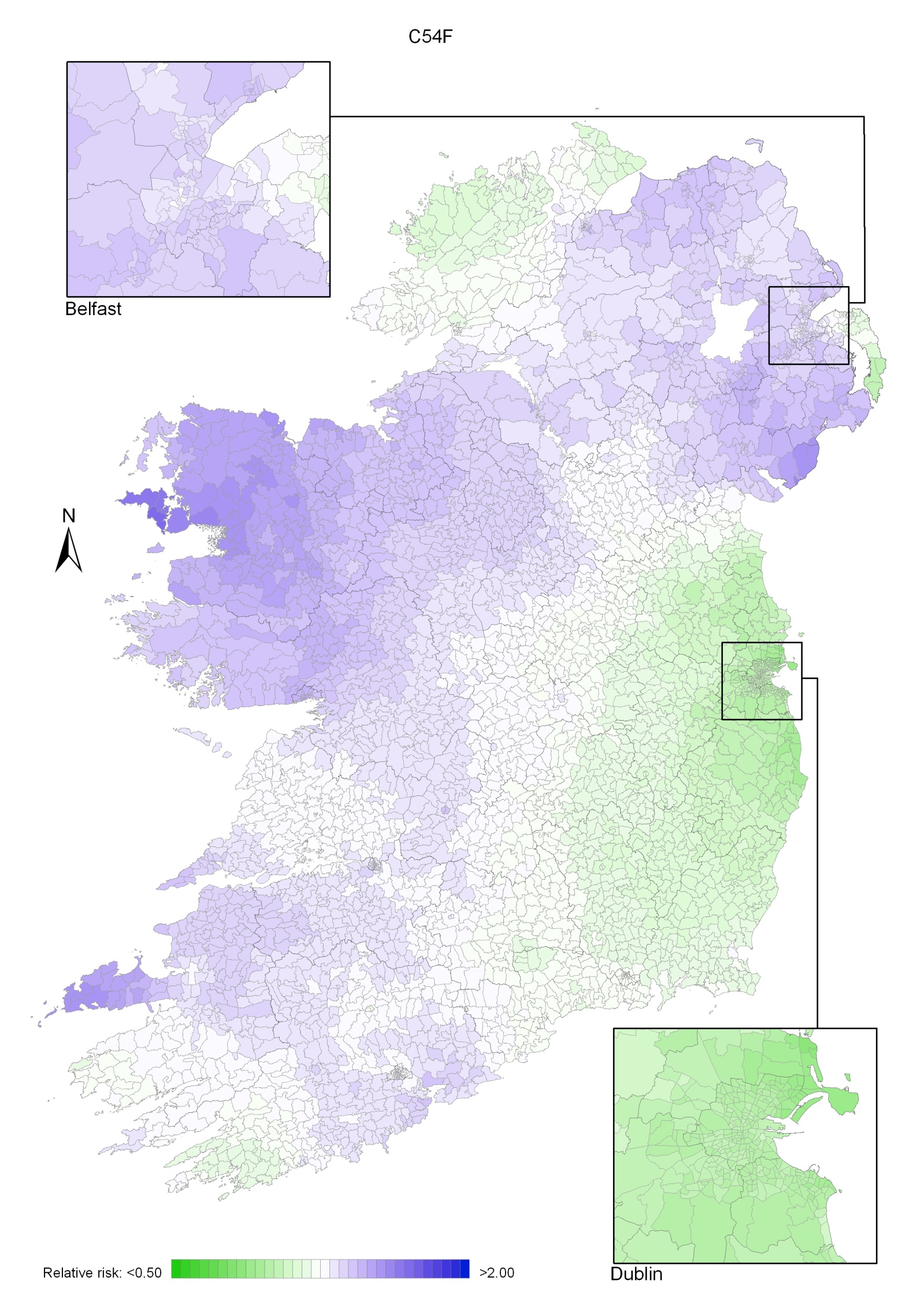
Uterine cancer had an unusual geographical pattern, with higher relative risk in NI, Connacht and parts of Munster (Map 19.1). Donegal and most of the east coast, south of the border with NI and including Dublin, had a lower relative risk.
Map 19.1 Cancer of the corpus uteri, smoothed relative risks